Session 1:
Overview of Pharmacokinetic Models and Computational Toolkits
Nan-Hung Hsieh, PhD
Postdoc @ Texas A&M Superfund Decision Science Core
12/09/2019
About Me
MS in Safety, Health and Environmental Engineering @ National United University
PhD in Bioenvironmental Systems Engineering @ National Taiwan University
Research Associate @ Institute of Labor, Occupational Safety And Health, Ministry of Labor
Postdoctoral Research Associate @ Texas A&M University
Associate Toxicologist @ California Environmental Protection Agency
My Research: Computational Toxicology & Risk Assessment
Interest: Software Development
Content
1 Basic Pharmacokinetics Concepts
2 Pharmacokinetic Models
3 Computational Toolkits
4 Hands-on Exercise
Basic Pharmacokinetics Concepts
Why We Need to Do
Computational Modeling
in Toxicology?
Why We Need to Do
Computational Modeling
in Toxicology?
"Prediction"
Pharmacokinetics (PK) / Toxiccokinetics (TK)
The study of the movement of chemicals in and out of the body (“what the body does to the chemical”)
ADME process
Absorption - How will it get in?
Distribution - Which tissue organ it will go?
Metabolism - How is it broken down and transformation?
Excretion - How it leave the body?
Kinetics: rates of change
PK is focus on TIME (t) and CONCENTRATION (C)
Pharmacokinetics / Pharmacodynamics
Pharmacokinetics is the study of the fate of chemical in a living organism through ADME process (absorption, distribution, metabolism, and elimination).
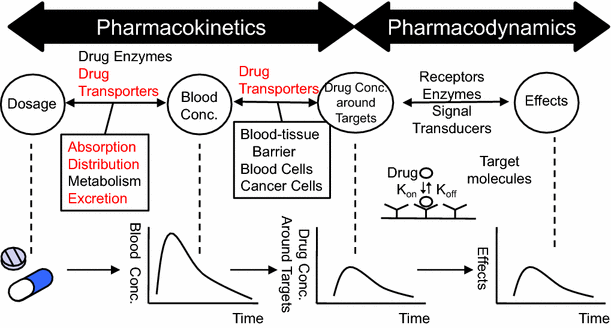
"PK" focus on DOSE to CONCENTRATION / "PD" focus on Concentration to Response
Pharmacokinetics profile

t: Time
C: Concentration
Cmax: The peak concentration
tmax: Time to reach Cmax
t1/2: Elimination half- life
AUC: Area under the curve
During the continous dose administered
Css: The averaged concentration
Cmin: The lowest concentration that a drug reaches
https://en.wikipedia.org/wiki/Pharmacokinetics
Absorption
Movement of chemical into the body
- Rate of absorption (how quickly does it absorb?)
- Fraction of absorption (how percentage does it absorb?)
Bioavailability ia a key factor for substance becomes available to the target tissue after administration.
Exposure routes
- Inhalation – alveolar gas exchange
- Oral – gut absorption
- Dermal – permeation through skin
- Intravenous
Different exposure route can related to different PK profile
Distribution
Transfer of the chemical between general circulation and tissues
Important toxicologically because tissue concentration drives toxicity
Factors affecting tissue distribution
- Blood flow to tissues
Tissue distribution (For chemicals that rapidly diffuse through membranes)
- Assume rapid equilibrium between blood and tissue
- Ratio is the tissue:blood partition coefficient
(Partition coefficient = Concentration in tissue / Concentration in blood) - Delivery to tissue limited by blood flow (“flow-limited”).
Metabolism/transformation
Enzyme systems, etc. covered in separate class.
- Phase I – conversion to more polar metabolite, e.g., oxidation by cytochrome P450
- Phase II – conjugation with an endogenous substrate to increase solubility, e.g., GSH conjugation, glucuronidation
Key issues relevant to risk assessment
- What are the metabolites?
- In what tissues does metabolism occur?
- What are the rates of metabolism?
- What is known about interspecies and intraspecies differences?
Metabolism process
- First order kinetics (linear; constant)
- Michaelis-Menten Kinetics (Non-linear; concentration dependent)
Metabolism - Acetaminophen

- Acetaminophen (APAP) is a widely used pain reliver and fever reducer.
- The therapeutic index (ratio of toxic to therapeutic doses) is unusually small.
- Phase I (APAP to NAPQI) is toxicity pathway at high dose.
- Phase II (APAP to APAP-Glucuronidation & APAP to APAP-Sulfation) is major pathways at therapeutic dose.
Trichloroethylene GSH conjugation pathway
Initial conjugation with GSH
Subsequent biotransformation occurs in multiple tissues via both Phase I and Phase II metabolism


Lash, L.H., et al. 2014. Trichloroethylene biotransformation and its role in mutagenicity.
Excretion
Excretion is the removal from the body
- Urine
- Exhalation
- Feces
- Minor pathways: sweat, saliva, milk
Excretion and elimination are often confused
- Excretion is reserved for exiting the body
- Elimination is the disappearance of a chemical, including both excretion and metabolism
Elimination kinetics
Zero-order kinetics
The elimination is independent with internal chemical dose
First-order kinetics
The elimination is dependent with internal chemical dose

Impact of TK on risk assessment
Concentration prediction
Identifying the toxicologically active agent(s)
Providing a basis for extrapolating from experimental studies to humans
Characterizing how humans may vary in their response to exposure due to differences in internal dose
Pharmacokinetic Models
Why Pharmacokinetic Modeling
Pharmaceutical research
Drug development
Health risk assessment
...
High Throughput Risk Prioritization
According to Toxic Substances Control Act (TSCA), to address thousands of chemicals, we need to use new approach methodologies (NAMs) to prioritize the existing and new chemicals for testing.
Main components
1. High throughput hazard characterization
2. High throughput exposure forecasts
3. High throughput toxicokinetics

source: USEPA
Pharmacokinetic Model
What information we want to know through "prediction"?
Time
- When the chemical can reach the peak concentration in the body?
- How long it stay in the body?
Concentration
- What is the peak concentration?
- What is the average concentration?
Mathematical method
How to conduct simulation?
Numerical
- Use the differential equation containing one or more functions of one independent variable and the derivatives of those functions.
dAdt=−ke⋅A
Analytical
- The simple equation with a independent variable of time
A(t)=A0⋅e−ke⋅t
A: Amount in body (mass); A0 initial amount (mass); t: time; ke: Elimination rate constant (/time)
The basic meaning of elimination rate is that the decreasing speed is the propotion of current concentration.
Intravenous administration
Numerical
dAdt=−ke⋅A
Analytical
At=A0⋅e−ke⋅t
A_0 <- 10t <- c(0,1,2,4,8,10)k_e <- 0.5A_t <- A_0*exp(-k_e*t) plot(t, A_t, type = "b")Oral administration

C(t)=F⋅D⋅kaV(ka−ke)⋅(e−ket−e−kat)
D: Dose (mass)
F: Bioavailability (-)
Ka Absorption rate constant (/time)
ke Elimination rate constant (/time)
V: Distribution Volume (Vol)
Empirical compartmental models
Advantages
Can continue adding compartments to fit more complex concentration-time profiles.
Can provide useful descriptions of the data.
Disadvantages
- Cannot interpret compartments biologically.
- Many A,D,M, and E processes are interconnected.
- Cannot make predictions beyond the experiment being described.
- Different tissues
- Different exposure routes or conditions
- Different species
- Different individuals
- Cannot incorporate everything else we know about biology!
Example pf empirical compartmental models
Luo, Y.S., Hsieh, N.H., Soldatow, V.Y., Chiu, W.A. and Rusyn, I., 2018. Comparative Analysis of Metabolism of Trichloroethylene and Tetrachloroethylene among Mouse Tissues and Strains. Toxicology, 409, pp.33-43.

Physiological-Based Pharmacokinetic (PBPK)
Mathematically transcribing anatomical, physiological, physical, and chemical descriptions of the phenomena involved in the complex ADME processes.

Why PBPK models?
There are some processes that empirical models have difficulty simulating – such as inhalation exposures.
We know a lot about the physiology/anatomy of the organisms we study and want to protect.
- Organ/tissue sizes
- Connections between organs/tissues
- Blood flow rates
- Ventilation rates
- Metabolizing enzymes
- Etc…
By developing a model, we can incorporate that information to help us make inferences beyond the experimental data at hand.
Building a PBPK model
Decide on what compartments are needed
- Target vs. non-target tissues
- Tissues with in vivo vs. in vitro vs. no data
- Exposure routes
Specify the (interrelated) equations for each compartment
Specify parameter values
- Physiological/anatomical parameters
- Chemical-specific parameters
Set up the inputs (doses, exposure concentrations, etc.) and outputs (blood, tissue, exhaled breath concentrations).
Solve using numerical differential equation solver and compare with data
Iteration may be needed for refining model and/or conducting additional experiments
PBPK Workflow
Model Construction
- What type of data we have?
- A: Input routes
- D: Blood and other tissues
- M: Metabolism
- E: Urine or feces
Parameter Estimates
- Do we have any prior information?
- Physiological parameter
- Chemical-specific parameter
PBPK modeling
Chen et al. (2015) Physiologically based pharmacokinetic modeling of zinc oxide nanoparticles and zinc nitrate in mice.
Compartmental equations
Flow in and out of “storage” compartments involve only blood flow
Key parameters/variables
Qi = tissue blood flow
Pi = tissue-blood partition coefficient
Vi = volume of tissue
Ca = arterial blood concentration
CVi = venous blood concentration leaving tissue
Ai = amount in tissue
Ci = concentration in tissue


Basic transport equations
The model equations follow the principles of mass transport, fluid dynamics, and biochemistry in order to simulate the fate of a substance in the body.
rate in =QiCart & rate out =QiCVT=Qi⋅Ci/Pi (Unit: mass/time)
Change in amount = rate in – rate out
dAidt=Qi(Cart−AiPiVi)
or
dCidt=QiVi(Cart−CiPiVi)
Ai is amount of chemical, Qi is blood flow, Cart incoming arterial blood concentration, Pi the tissue over blood partition coefficient, and Vi the volume of compartment i.

Different exposure routes
Inhalation
Cart=Qpul(1−rds)Cinh+QtotCvenQpul(1−rds)/Pair+Qtot
Ingestion
dAivdt=Qliv(Cart−AlivPlivVliv)−kmetAliv+Ring
A: quantity; Q: flow rate; rds: deadspace ratio; C: concentration; Ring: administration rate; P partition coefficient; k rate constant
Bois F.Y., Brochot C. (2016) Modeling Pharmacokinetics. In: Benfenati E. (eds) In Silico Methods for Predicting Drug Toxicity. Methods in Molecular Biology
Computational toolkits
Computational toolkits
Multiple software packages available to solve differential equations
- ASCL – “traditionally” used by many established PBPK modelers (but now discontinued!)
- Berkeley Madonna – used in some PBPK modeling courses
- MatLab – flexible interactive interface (more expensive, but more powerful)
- GNU MCSim – specialized software for Bayesian uncertainty and population variability analyses
All of them incorporate methods for “optimizing” the model fit by adjusting parameter values.
Computational toolkits
GNU MCSim
Simulation package, which allows you to:
design and run simulation models (using algebraic or differential equations)
perform Monte Carlo stochastic simulations
do Bayesian inference through Markov Chain Monte Carlo simulations
has faster computing speed than other simulation software/packages (e.g., Asclx, Berkeley Madonna, RStan)
R
Programming language that allows you to:
conduct statistical analysis (summarization, estimation)
visualize simulation results
use various packages to analyze results (e.g., CODA, BOA, rstan)
perform sensitivity analysis (e.g., sensitivity, pksensi)
access community support (e.g., Stack Overflow, R User groups)
Advantages of MCSim and R
"Free and Open Source Software" under GNU General Public License
- The freedom to run the program as you wish, for any purpose (freedom 0).
- The freedom to study how the program works, and change it so it does your computing as you wish (freedom 1). Access to the source code is a precondition for this.
- The freedom to redistribute copies so you can help others (freedom 2).
- The freedom to distribute copies of your modified versions to others (freedom 3). By doing this you can give the whole community a chance to benefit from your changes. Access to the source code is a precondition for this.
Advantages
"Parallel computing"
- Run multiple MCMC chains with multiple CPUs
- High performance (cloud) computing
Advantages - Reproducible research

Reproducibility and Replicability in Science (2019) http://nap.edu/25303
Conducting data analysis through R
Implement a wide variety of statistical and graphical techniques
Comprehensive research workflow and toolkits (e.g., RMarkdown)
Integration with low-level language (e.g., C, C++, Fortran)
Highly extensible through the use of user-submitted packages
Webapp development (e.g., http://webpopix.org/shiny/ShinyExamples.html)
RStudio
Free and open-source integrated development environment
Powerful and user friendly programming interface
Designed to make it easy to write scripts
Easy to view and interact with the objects
R project with version control (e.g., git)
Support cloud computing https://rstudio.cloud/

GNU MCSim
- The project started by Don Maszle and Frederic Y. Bois in UC Berkeley, 1991. It is written in standard C language as a model preprocessor.
- First public release in 1993 (straight simulations with Monte Carlo modeling).
GNU MCSim is a general purpose modeling and simulation program which can performs "standard" or "Markov chain" Monte Carlo simulations. It allows you to specify a set of linear or nonlinear algebraic equations or ordinary differential equations. They are solved numerically using parameter values you choose or parameter values sampled from statistical distributions. Simulation outputs can be compared to experimental data for Bayesian parameter estimation (model calibration).
- 6.1.0 (19 February 2019)
- 6.0.1 (05 May 2018)
- 6.0.0 (24 February 2018)
- 5.6.6 (21 January 2017)
- 5.6.5 (27 February 2016)
- 5.6.4 (30 January 2016)
- 5.6.3 (1 January 2016)
- 5.6.2 (24 December 2015)
- 5.6.1 (21 December 2015)
- 5.6.0 (16 December 2015)
- 5.5.0 (17 March 2013)
- 5.4.0 (18 January 2011)
- 5.3.1 (3 March 2009)
- 5.3.0 (12 January 2009)
- 5.2 beta (29 January 2008)
- 5.1 beta (18 September 2006)
- 5.0.0 (4 January 2005)
- 4.2.0 (15 October 2001)
- 4.1.0 (1 August 1997)
- 4.0.0 (24 March 1997)
Founder: Frédéric Y. Bois
Staff Toxicologist (Specialist),
Reproductive and Cancer Hazard Assessment Section,
CalEPA, Berkeley, USA, 1991-96
This project mainly focus on using GNU MCSim
This founder of this project is Dr. Frédéric Y. Bois. He had been worked in CalEPA as Staff Toxicologist for 5 years.
GNU MCSim Overview
The GNU MCSim consists in two pieces, a model generator and a simulation engine:
The model generator, "mod"
- Created to facilitate structural model definition and maintenance, while keeping execution time short. You can code your model using a simplified syntax and use mod to translate it to
C(model.c).
The simulation engine, "sim"
- A set of routines which are linked to your model during compilation to produce executable program (
mcsim.model). After that, you can run simulations of your model under a variety of conditions, specify an associated statistical model, and perform simulations.
GNU MCSim Workflow

Types of Simulation
Simple simulation
- Straight simulations (set parameter values and initial conditions).
Used to: Model testing when building the model (e.g., mass balance)
Monte Carlo simulations
- Perform repeated (stochastic) simulations across a randomly sampled region of the model parameter space.
Used to: Check possible simulation (under given parameter distributions) results before model calibration
SetPoints simulation
- Solves the model for a series of specified parameter sets. You can create these parameter sets yourself or use the output of a previous Monte Carlo or MCMC simulation.
Used to: Posterior analysis, Local/global sensitivity analysis
Types of Simulation
Markov-chain Monte Carlo (MCMC) simulation
- Performs a series of simulations along a Markov chain in the model parameter space.
- They can be used to obtain the Bayesian posterior distribution of the model parameters, given a statistical model, prior parameter distributions and data for which a likelihood function can be computed.
- GNU MCSim can handle hierarchical statistical models as well.
MCSim-related R packages
httk: R Package for High-Throughput Toxicokinetics
Robert G. Pearce, R. Woodrow Setzer, Cory L. Strope, Nisha S. Sipes, John F. Wambaugh
MCSim (Bois and Maszle 1997) was used for converting the model equations into C code, which is used with deSolve (Soetaert et al. 2016) in solving each system of equations.
Journal of Statistical Software; http://dx.doi.org/10.18637/jss.v079.i04
GNU MCSim model code C code deSolve package Prediction
MCSim-related R packages
pksensi: R Package for Global Sensitivity Analysis in Pharmacokinetic Modeling
Nan-Hung Hsieh, Brad Reisfeld, Weihsueh A. Chiu
pksensi implements the global sensitivity analysis workflow to investigate the parameter uncertainty and sensitivity in pharmacokinetic (PK) models, especially the physiologically based pharmacokinetic (PBPK) model with multivariate outputs.
Two types of model solver
solve_fun()
GNU MCSim model code
C code
deSolve package
Prediction
solve_mcsim()
GNU MCSim model code
Prediction
Note: solve_mcsim() is faster than solve_fun()
Disadvantages in MCSim and R
Difficult learning curve (command line interface-based)
Requires coding/programming skill
Requires installation of extra program or package
Requires "debugging"
Git
- Manage your code (models, inputs, R script)
- Transfer your file (through GitHub or GitLab)
- Collaboration work

GitHub

High Performance Research Computing
The efficient way to conduct parallel computing

Linux
The open source Unix-like operating systems for high performance research computing

Summary
Basic pharmacokinetics
- A,D,M,E each may affect internal dose, and differences between species or among individuals.
Role of pharmacokinetics in risk assessment
- Exposure / concentration prediction
Pharmacokinetic modelling
- Information from physiology and chemistry are common ways to characterize the concentration-time relationship.
Computational toolkits
- The open soure computational tools (e.g., R & GNU MCSim) are powerful and can help us conduct the model simulation and data analysis
Hands on Exercise
Hands on Exercise
Task 1. Exploratory analysis of PK data (code: https://rpubs.com/Nanhung/SRP19_1)
- Learn how to use R to conduct basic analysis for PK data
Task 2. PK model development (code: https://rpubs.com/Nanhung/SRP19_2)
- Learn how to use R and MCSim to build a model
Task 3. Parameter setting and model simulation (code https://rpubs.com/Nanhung/SRP19_3)
- Understand the parameter seting in PK model and conduct the simulation
Task 4. PBPK model development (code https://rpubs.com/Nanhung/SRP19_4)
- Instead of PK model, we need to know how to build a PBPK model
Task 5. Application of PBPK model (code https://rpubs.com/Nanhung/SRP19_5)
- Here, we have a well-built PBPK model and its parameters, let's apply the model in the exposure assessment
Hands on Exercise
Task 1: Exploratory analysis of PK data
- Now, we have a theophylline PK dataset. The purpose of this exercise is to develop the simple PK model and use it to describe the PK of theophylline. First, look into the theophylline dataset. The
Theophdata frame has 132 rows and 5 columns of data from an experiment on the pharmacokinetics of theophylline. Then, find the Cmax and Tmax for each individual.
head(Theoph)## Subject Wt Dose Time conc## 1 1 79.6 4.02 0.00 0.74## 2 1 79.6 4.02 0.25 2.84## 3 1 79.6 4.02 0.57 6.57## 4 1 79.6 4.02 1.12 10.50## 5 1 79.6 4.02 2.02 9.66## 6 1 79.6 4.02 3.82 8.58- Plot the pharmacokinetic diagram for each individual.
Hands on Exercise
Task 2: PK model development
- Develop the non compartment model and compartment model in R and MCSim
- Non compartment model
C(t)=F⋅D⋅kaV(ka−ke)⋅(e−ket−e−kat)
- Compartment model
dAgutdt=−kaAgut
dAdt=kaAgut−keAe C=A/V
Hands on Exercise
Task 3: Parameter setting and simulation
Extract the chemical information from httk package
library(httk)parms <- httk::parameterize_1comp(chem.name = "theophylline")Use the parameters in the developed model and conduct the simulation
- Compare the difference between data and the model simulation result (Cmax, Tmax)
Hands on Exercise
Task 4: PBPK model development
Instead of simple PK model, in this exercise we want to apply the well developed PBPK model for 1,3 butadiene.
First, reproduce the simulation result from the published paper*.
[*] Bois F.Y., Brochot C. (2016) Modeling Pharmacokinetics. In: Benfenati E. (eds) In Silico Methods for Predicting Drug Toxicity. Methods in Molecular Biology, vol 1425. Humana Press, New York, NY
Inputs
C_inh <- approxfun(x = c(0, 120), y=c(10,0), method = "constant", f = 0, rule = 2)plot(C_inh(1:300), type="l", xlab = "Time (min)", ylab = "Butadiene inhaled concentration (ppm)")Parameters and outputs
parameters <- c("BDM" = 73, # Body mass (kg) "Height" = 1.6, # Body height (m) "Age" = 40, # in years "Sex" = 1, # code 1 is male, 2 is female "Flow_pul" = 5, # Pulmonary ventilation rate (L/min) "Pct_Deadspace" = 0.7, # Fraction of pulmonary deadspace "Vent_Perf" = 1.14, # Ventilation over perfusion ratio "Pct_LBDM_wp" = 0.2, # wp tissue as fraction of lean mass "Pct_Flow_fat" = 0.1, # Fraction of cardiac output to fat "Pct_Flow_pp" = 0.35, # ~ to pp "PC_art" = 2, # Blood/air partition coefficient "PC_fat" = 22, # Fat/blood ~ "PC_wp" = 0.8, # wp/blood ~ "PC_pp" = 0.8, # pp/blood ~ "Kmetwp" = 0.25) # Rate constant for metabolismy <- c("Q_fat" = 0, # Quantity of butadiene in fat (mg) "Q_wp" = 0, # ~ in well-perfused (mg) "Q_pp" = 0, # ~ in poorly-perfused (mg) "Q_met" = 0) # ~ metabolized (mg)Model structure
# Define the model equationsbd.model <- function(t, y, parameters) { with (as.list(y), { with (as.list(parameters), { # Define constants # Calculate flow and volumes # Calculate the tissue, blood, and air # Differentials for quantities # The function bd.model must return at least the derivatives list(c(dQ_fat, dQ_wp, dQ_pp, dQ_met), # derivatives c("C_ven" = C_ven, "C_art" = C_art)) # extra outputs }) # end with parameters }) # end with y} # end bd.modelConstants
Known constants
# Known constantsHeight = 1.6 # use to calculate fraction of body fatAge = 40 # use to calculate fraction of body fatSex = 1 # use to calculate fraction of body fatMW_bu = 54.0914 # butadiene molecular weight (in grams)Conversions from/to ppm
ppm_per_mM = 24450 # ppm to mM under normal conditionsppm_per_mg_per_l = ppm_per_mM / MW_bumg_per_l_per_ppm = 1 / ppm_per_mg_per_lFlows and volumes
Air and blood flow
# Calculate Flow_alv from total pulmonary flowFlow_alv = Flow_pul * (1 - Pct_Deadspace)# Calculate total blood flow from Flow_alv and the V/P ratioFlow_tot = Flow_alv / Vent_Perf# Calculate fraction of body fatPct_BDM_fat = (1.2 * BDM / (Height * Height) - 10.8 *(2 - Sex) + 0.23 * Age - 5.4) * 0.01# Calculate actual blood flows from total flow and percent flowsFlow_fat = Pct_Flow_fat * Flow_totFlow_pp = Pct_Flow_pp * Flow_totFlow_wp = Flow_tot * (1 - Pct_Flow_pp - Pct_Flow_fat)Volumes
# Actual volumes, 10% of body mass (bones…) get no butadieneEff_V_fat = Pct_BDM_fat * BDMEff_V_wp = Pct_LBDM_wp * BDM * (1 - Pct_BDM_fat)Eff_V_pp = 0.9 * BDM - Eff_V_fat - Eff_V_wpConcentrations - tissues & blood
# Calculate the concentrationsC_fat = Q_fat / Eff_V_fatC_wp = Q_wp / Eff_V_wpC_pp = Q_pp / Eff_V_pp# Venous blood concentrations at the organ exitCout_fat = C_fat / PC_fatCout_wp = C_wp / PC_wpCout_pp = C_pp / PC_pp# Sum of Flow * Concentration for all compartmentsdQ_ven = Flow_fat * Cout_fat + Flow_wp * Cout_wp + Flow_pp * Cout_ppC_inh.current = C_inh(t) # to avoid calling C_inh() twice# Arterial blood concentration# Convert input given in ppm to mg/l to match other unitsC_art = (Flow_alv * C_inh.current * mg_per_l_per_ppm + dQ_ven) / (Flow_tot + Flow_alv / PC_art)# Venous blood concentration (mg/L)C_ven = dQ_ven / Flow_totConcentrations - air
# Alveolar air concentration (mg/L)C_alv = C_art / PC_art# Exhaled air concentration (ppm)if (C_alv <= 0) { C_exh = 10E-30 # avoid round off errors} else { C_exh = (1 - Pct_Deadspace) * C_alv * ppm_per_mg_per_l + Pct_Deadspace * C_inh.current}Differentials for quantities
# Quantity metabolized in liver (included in well-perfused)dQmet_wp = Kmetwp * Q_wp# Differentials for quantitiesdQ_fat = Flow_fat * (C_art - Cout_fat)dQ_wp = Flow_wp * (C_art - Cout_wp) - dQmet_wpdQ_pp = Flow_pp * (C_art - Cout_pp)dQ_met = dQmet_wpOutputs
# Define the computation output timest <- seq(from=0, to=1440, by=10)# Solve ODElibrary(deSolve)out <- ode(times=t, func=bd.model, y=y, parms=parameters)head(out)## time Q_fat Q_wp Q_pp Q_met C_ven C_art## [1,] 0 0.00000000 0.00000000 0.00000000 0.00000000 0.000000000 0.01606403## [2,] 10 0.02293618 0.03724892 0.07427798 0.06645654 0.003338318 0.01819035## [3,] 20 0.04722954 0.04026245 0.14189019 0.16431358 0.004379098 0.01885327## [4,] 30 0.07221315 0.04152080 0.20176415 0.26661643 0.005210310 0.01938270## [5,] 40 0.09777386 0.04256838 0.25471138 0.37175647 0.005941936 0.01984870## [6,] 50 0.12383371 0.04349410 0.30153555 0.47935418 0.006589697 0.02026129plot(out)Hands on Exercise
Task 5: Apply the PBPK model
- Based on the OSHA permissible exposure limit (PEL), the time-weighted average is 1 ppm. If a worker is under the exposure for a long time, what is the estimated blood concentration.

